Fluorescence Imaging Filters

- Excitation, Emission, and Dichroic Filters for Imaging Applications
- Matched Filter Sets Designed for Wavelength Ranges of Common Fluorophores
MD568
Dichroic Mirror
MDF-TOM
Filter Set
MF630-69
Emission Filter
Arrow Indicates
Direction of Light
Propagation
Etched Corner Indicates Dichroic-Coated Side
Exploded View of a Filter Set Installed Into a Filter Cube
Dichroic
Mirror
Inside Cube
Excitation
Filter
Emission
Filter
Image Plane
Retaining
Ring
Retaining
Ring

Please Wait

Excitation and emission filters are marked with an arrow that shows the recommended direction of light propagation.

Dichroic filters are marked on the side with the dichroic coating. Light should be incident on this side for best performance.
Features
- Excitation, Emission, and Dichroic Filters for Fluorescence Imaging
- >90% Transmission over Desired Wavelength Band
- Sharp Cutoff (T < 0.001%) Outside the Transmission Band
- Matched Filter Sets Designed to Target Many Common Fluorophores
(See Fluorophores Tab for Alternate Fluorophore Compatibility)- BFP
- CFP
- WGFP
- GFP- FITC
- Alexa Fluor® 488
- YFP
- tdTomato- TRITC
- Texas Red
- mCherry
- Cyanine (CY3.5) - Excitation and Emission Filters are Mounted in Black-Anodized Housings
- Dichroic Filters are Unmounted
- Filter Sets are Available at a Savings Over Individual Filter Prices
These excitation, emission, and dichroic filters are designed specifically for use in fluorescence imaging applications. They are fabricated at industry-standard dimensions that make them compatible with filter cubes from all major manufacturers. We offer individual filters and filter sets targeted at common fluorophores: BFP, CFP, WGFP, GFP, FITC, Alexa Fluor® 488, YFP, tdTomato, TRITC, Texas Red, mCherry, and Cyanine (CY3.5). In addition, the Fluorophores tab provides information on the alternative fluorophores suitable for these filters. These filters are also available pre-installed into our microscope filter cubes.
Filter Design
Our filters are manufactured to high-performance optical specifications and designed for durability. They are produced via multiple dielectric layers deposited on a high-precision, fused silica substrate. The substrate is ground and polished to ensure that the highest possible image quality is maintained. The resulting hard-coated optics consist of filter layers that are denser than those obtained from electron beam deposition techniques, and which reduce water absorption while greatly enhancing durability, stability, and performance of the filter. Each filter layer is monitored during growth to ensure minimal deviation from design specification thickness, ensuring overall high-quality filter performance.
Each excitation or emission filter is housed in a Ø25 mm black anodized aluminum ring which makes handling easier and enhances the blocking OD by limiting scattering. These filters can be mounted in our extensive line of filter mounts and wheels. As the aluminum rings are not threaded, Ø1" retaining rings will be required to mount the Ø25 mm filters in one of our internally-threaded SM1 lens tubes. For customers who wish to use these filters in Thorlabs, Olympus, or Nikon fluorescence microscopes, Thorlabs manufactures a family of Drop-In Microscope Filter Cubes. Additionally, the unmounted 25 mm x 36 mm dichroic filters can be mounted in the KM2536 kinematic mount, which is designed to secure a 1 mm thick rectangular optic with minimal stress.
| Specification | Excitation Filters | Emission Filters | Dichroic Filters | |||
|---|---|---|---|---|---|---|
| MDF-GFP2, MDF-TOM, MDF-MCHC, & MDF-MCHA Kits |
All Other Excitation Filters |
MDF-GFP2, MDF-TOM, MDF-MCHC, & MDF-MCHA Kits |
All Other Excitation Filters |
Dichroics in MDF-GFP2, MDF-MCHA, MDF-MCHC, & MDF-TOM Kits |
All Other Dichroics | |
| Size | Ø25 +0.0/-0.1 mm | Ø25 ± 0.1 mm | Ø25 +0.0/-0.1 mm | Ø25 ± 0.1 mm | 25.2 mm x 35.6 mm | 25.0 mm x 36.0 mm |
| Clear Aperture | >Ø21 mm | >Ø22 mm | >Ø21 mm | 80% of Area | >(22.5 mm x 32.4 mm) | |
| Angle of Incidence | 0° ± 5° | 45° ± 1.5° | ||||
| Thickness | 5.0 ± 0.1 mm | 3.5 ± 0.1 mm | 1.05 ± 0.05 mm | 1.0 ± 0.1 mm | ||
| Surface Quality | 60-40 Scratch-Dig | |||||
| Substrate | Fused Silica | |||||
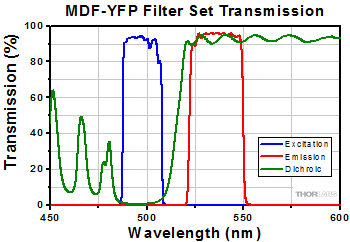
Click to Enlarge
MDF-YFP filter set transmission graph. Note the dichroic mirror (green) reflects light in the excitation wavelength range (blue), and transmits light in the emission wavelength range (green).
Filters for Fluorescence Microscopy
Fluorophores
A fluorophore is a molecule or portion of a molecule that is capable of producing fluorescence. When light of the appropriate frequency necessary to excite a molecule from its ground state to an excited state is present, excitation will occur. However, once in an excited state, the molecule will be unstable. After some short period of time (typically 10-15 to 10-9 s), a photon will be released, thereby enabling the molecule to return to a lower energy state. The emitted radiation will be at a longer wavelength (lower energy) than the absorbed radiation due to the loss of energy through various mechanisms such as vibrations, sound, and thermal energy.
A single fluorophore can be continually excited unless it is destroyed by photobleaching (i.e. the nonreversible destruction of a fluorophore due to photon-induced chemical damage or covalent modification). The average number of excitation and emission cycles that a particular fluorophore can undergo prior to photobleaching depends on its molecular structure and the local environment; some fluorophores bleach quickly after emitting only a few photons while others are far more robust and can undergo thousands or even millions of cycles before bleaching occurs.
Filters for Fluorescence Microscopy
The experimental setup to the right shows the typical filters used for epi-fluorescence microscopy, a form of microscopy in which both the excitation and emission light travel through the microscope objective. By carefully choosing the appropriate filters and mirrors for a given application, the signal-to-noise ratio can be maximized. As shown in the schematic to the right, three types of filters are used to maximize the fluorescence signal while minimizing the unwanted radiation. Each optical element is discussed below.
Excitation Filter
The excitation filter only allows a narrow band of wavelengths to pass through it, around the peak fluorophore excitation wavelength. For example, as shown in the graph to the right, the bandpass region corresponding to greater than 90% transmission for the Yellow Fluorescent Protein (YFP) Excitation Filter (MF497-16) is 489 - 505 nm; incident radiation outside of this range is either partially (for regions near the transmission region) or totally (for regions further from the bandpass region) blocked by the filter.
Dichroic Mirror
Dichroic mirrors are designed to reflect light whose wavelength is below a specific value (i.e. the cutoff wavelength) while permitting all other wavelengths to pass through it unaltered. In a microscope, the dichroic mirror directs the proper wavelength range to the sample as well as to the image plane. The cutoff wavelength value associated with each mirror indicates the wavelength that corresponds to 50% transmission. For example, as shown in the graph to the right, the cutoff wavelength for the Yellow Fluorescent Protein (YFP) Dichroic Mirror (MD515) is ~515 nm. The Specs tab provides information on the reflectance and transmission for each type of dichroic mirror.
By placing one of these mirrors into the experimental setup at 45° with respect to the incident radiation, the excitation radiation (shown in blue in the above right schematic) is reflected off of the surface of the dichroic mirror and directed towards the sample and microscope objective, while the fluorescence emanating from the sample (shown in red in the above right schematic) passes through the mirror to the detection system.
Although dichroic mirrors play a crucial role in fluorescence microscopy, they are not perfect when it comes to blocking unwanted light; typically, ~90% of the light at wavelengths below the cutoff wavelength value are reflected and ~90% of the light at wavelengths above this value are transmitted by the dichroic mirror. Hence, some of the excitation light can be transmitted through the dichroic mirror along with the longer wavelength fluorescence emitted by the sample. To prevent this unwanted light from reaching the detection system, an emission filter is used in addition to the dichroic mirror.
Emission Filter
An emission filter serves the purpose of allowing the desirable fluorescence from the sample to reach the detector while blocking unwanted traces of excitation light. Like the excitation filter, this filter only allows a narrow band of wavelengths to pass through it, around the peak fluorophore emission wavelength. For example, as shown in the graph to the right, the bandpass region corresponding to greater than 90% transmission for the Yellow Fluorescent Protein (YFP) Emission Filter (MF535-22) is 524 - 546 nm; incident radiation outside of this range is either partially (for regions near the transmission region) or totally (for regions further from the bandpass region) blocked by the filter.
| Keya | |
|---|---|
 |
Acceptable - Actual performance depends on individual experimental conditions. |
  |
Compatible - Ideal, or nearly ideal, performance under most experimental conditions. |
   |
Optimized - Optimal performance under all experimental conditions. |
The table below displays all of the fluorophores that are compatible with our filter sets. The filter set item numbers are listed across the top row and the fluorophores are listed down the first column. Scroll through the table to view fluorophore compatibility with our filter sets.
Click on the  below to view the filter set transmission with the absorption and emission spectra of the fluorophore. The key to the right details the meaning of all check marks
below to view the filter set transmission with the absorption and emission spectra of the fluorophore. The key to the right details the meaning of all check marks  in the table below. Please note that absorption and emission spectra are unavailable if any
in the table below. Please note that absorption and emission spectra are unavailable if any  is red.
is red.
| 1,8-ANS (372 nm, 477 nm) | ✓ | ||||||||||||
| 2-dodecylresorufin-lipid (582 nm, 595 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | |||||||
| 5-carboxy-2,7-dichlorofluorescein (500 nm, 525 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| 5-carboxynapthofluorescein (pH 10) (555 nm, 615 nm) | ✓ | ✓ | |||||||||||
| 5-FAM (5-carboxyfluorescein) (492 nm, 518 nm) | ✓✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| 5-ROX (carboxy-X-rhodamine) (578 nm, 604 nm) | ✓ | ✓ | ✓✓✓ | ✓✓ | ✓✓ | ✓ | |||||||
| 5-TAMRA (5-carboxytetra- methylrhodamine, pH 7.0) (542 nm, 568 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| 6-carboxyrhodamine 6G (525 nm, 555 nm) | ✓ | ✓ | |||||||||||
| 6-JOE (520 nm, 548 nm) | ✓ | ✓ | |||||||||||
| 7-AAD (548 nm, 648 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Acridine Orange (502 nm, 526 nm) | ✓ | ✓ | ✓ | ||||||||||
| Acridine Yellow (470 nm , 550 nm) | ✓ | ✓✓ | ✓ | ✓ | |||||||||
| Alexa Fluor 350™ (343 nm, 441 nm) | ✓✓ | ||||||||||||
| Alexa Fluor 405™ (401 nm, 422 nm) | ✓ | ||||||||||||
| Alexa Fluor 430™ (431 nm , 541 nm) | ✓ | ✓ | ✓ | ||||||||||
| Alexa Fluor 488™ (499 nm, 520 nm) | ✓ | ✓✓✓ | ✓✓ | ||||||||||
| Alexa Fluor 500™ (503 nm, 525 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| Alexa Fluor 514™ (518 nm, 543 nm) | ✓ | ||||||||||||
| Alexa Fluor 546™ (556 nm, 572 nm) | ✓✓✓ | ✓ | |||||||||||
| Alexa Fluor 568™ (579 nm, 603 nm) | ✓ | ✓ | ✓✓✓ | ✓✓ | ✓✓ | ✓ | |||||||
| Alexa Fluor 594™ (590 nm, 618 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| AMCA (Aminomethylcoumarin) (350 nm, 488 nm) | ✓✓ | ||||||||||||
| AmCyan1 (458 nm, 489 nm) | ✓✓✓ | ✓✓ | ✓ | ✓ | |||||||||
| Amplex UltraRed peroxidation product -pH 7.5 (568 nm, 581 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Aqua 431 (430 nm,478 nm) | ✓✓ | ✓ | |||||||||||
| AsRed 2 (576 nm, 592 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| ATTO 390 (392 nm, 477 nm) | ✓✓ | ||||||||||||
| ATTO 425 (436 nm, 484 nm) | ✓✓ | ✓✓ | |||||||||||
| ATTO 465 (453 nm, 507 nm) | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓ | ||||||||
| ATTO 488 (500 nm, 525 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| ATTO 495 (495 nm, 527 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ||||||||
| ATTO 520 (516 nm, 538 nm) | ✓ | ✓ | |||||||||||
| ATTO 550 (553 nm, 576 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| ATTO 565 (563 nm, 592 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ||||||||
| ATTO 590 (594 nm, 624 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| ATTO 594 (601 nm, 624 nm) | ✓ | ||||||||||||
| BCECF (pH 5.5) (481 nm, 518 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| BCECF (pH 9.0) (502 nm, 528 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| BD Horizon V450 (406 nm, 449 nm) | ✓✓ | ||||||||||||
| BD Horizon V500 (410 nm, 500 nm) | ✓ | ✓ | ✓ | ||||||||||
| BFP (EBFP) (380 nm, 440 nm) | ✓✓ | ||||||||||||
| BOBO™-1 (461 nm, 484 nm) | ✓✓✓ | ✓✓ | ✓ | ✓ | |||||||||
| BOBO™-3 (570 nm, 605 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | |||||||
| BODIPY FL (505 nm, 512 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| BODIPY FL-MeOH (502 nm, 511 nm) | ✓ | ✓ | ✓ | ||||||||||
| BODIPY R6G (528 nm, 547 nm) | ✓ | ✓ | |||||||||||
| BODIPY TR-X phallacidin (590 nm, 621 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Calcein (494 nm, 514 nm) | ✓ | ✓✓ | ✓ | ✓ | |||||||||
| Calcium Crimson (589 nm, 609 nm) | ✓ | ✓ | |||||||||||
| Calcium Green-1 (507 nm, 529 nm) | ✓ | ✓✓✓ | |||||||||||
| Calcium Orange (589 nm, 609 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Carboxynaphthofluorescein (599 nm, 674 nm) | ✓ | ✓ | ✓ | ||||||||||
| Cascade Blue™ (401 nm, 419 nm) | ✓ | ||||||||||||
| CellTrace BODIPY TR methyl ester (598 nm, 625 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| CellTrace calcein violet (400 nm, 452 nm) | ✓✓ | ||||||||||||
| CellTracker Red CMTPX (586 nm, 614 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| CellTracker Violet BMQC+GSH (406 nm, 526 nm) | ✓ | ✓ | |||||||||||
| Cerulean (434 nm, 473 nm) | ✓✓✓ | ✓ | ✓ | ||||||||||
| CFP (ECFP) (433 nm, 475 nm) | ✓✓✓ | ✓ | ✓ | ✓ | |||||||||
| CFP2 (490 nm, 510 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| Cy2™ (492 nm, 507 nm) | ✓ | ✓✓✓ | ✓ | ||||||||||
| Cy3.5™ (578 nm, 591 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓✓ | ✓✓ | |||||||
| CyQUANT GR-DNA (502 nm, 523 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| DAF-FM-NO (495 nm, 519 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| DAPI (359 nm, 461 nm) | ✓✓✓ | ||||||||||||
| DDAO (648 nm, 657 nm) | ✓ | ✓ | ✓ | ||||||||||
| DEAC (432 nm, 472 nm) | ✓ | ✓✓ | ✓ | ||||||||||
| Dendra2 (Green) (491 nm, 507 nm) | ✓✓ | ✓✓ | ✓ | ||||||||||
| DiA (457 nm, 586 nm) | ✓ | ✓ | ✓ | ||||||||||
| DiA (457 nm, 586 nm) | ✓ | ✓ | |||||||||||
| DiO (475 nm, 500 nm) | ✓✓✓ | ✓✓ | ✓ | ✓ | |||||||||
| Dronpa (502 nm, 516 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| DsRed (559 nm, 583 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| DsRed-Express (556 nm, 584 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| dTomato (586 nm, 582 nm) | ✓✓✓ | ✓ | ✓ | ||||||||||
| DY-415 (418 nm, 470 nm) | ✓ | ✓ | ✓ | ||||||||||
| DY-505-Phalloidin (503 nm, 530 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| DY-590 (582 nm, 599 nm) | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | ||||||||
| DyLight 405 (398 nm, 420 nm) | ✓✓ | ||||||||||||
| DyLight 594 (593 nm, 618 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| ECFP (435 nm, 475 nm) | ✓ | ✓✓ | ✓ | ||||||||||
| ecliptic pHluorin pH 5.5 (473 nm, 507 nm) | ✓✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| Emerald (491 nm, 511 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| Eosin (525 nm, 546 nm) | ✓✓✓ | ✓✓ | |||||||||||
| ER-Tracker™ Blue-White DPX (372 nm, 557 nm) | ✓ | ||||||||||||
| Ethidium bromide (518 nm, 603 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Ethidium homodimer (527 nm, 617 nm) | ✓ | ✓✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| evoglow-Bs1 (449 nm, 496 nm) | ✓ | ✓✓ | ✓ | ||||||||||
| FITC (Fluorescein) (495 nm, 519 nm) | ✓ | ✓✓✓ | ✓✓ | ✓✓ | |||||||||
| FlAsH-CCPFCC (511 nm, 530 nm) | ✓ | ✓✓ | ✓ | ||||||||||
| Fluo-3 (506 nm, 527 nm) | ✓ | ✓✓✓ | |||||||||||
| Fluo-4 (494 nm, 516 nm) | ✓ | ✓✓✓ | ✓✓ | ||||||||||
| Fluorescein dextran (501 nm, 524 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| Fluorescein-pH 8.0 (489 nm, 517 nm) | ✓ | ✓✓ | ✓ | ✓ | |||||||||
| Fluoro-Emerald (494 nm, 518 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| FluoSpheres Crimson fluorescent microspheres (620 nm, 646 nm) | ✓ | ✓ | ✓ | ||||||||||
| FluoSpheres Red fluorescent microspheres 656 nm, 683 nm) | ✓ | ✓✓ | ✓✓ | ||||||||||
| FluoSpheres Yellow-Green fluorescent microspheres (503 nm, 514 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| FM 1-43 (473 nm, 579 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| GFP (EGFP) (489 nm, 511 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| Green 496 (496 nm, 520 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| Green 500 (501 nm, 524 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| HcRed1 (588 nm, 618 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| HCS LipidTOX Green neutral lipid stain (498 nm, 507 nm) | ✓✓ | ✓ | ✓ | ||||||||||
| HCS LipidTOX Green phospholipidosis (504 nm, 536 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| HCS LipidTOX Red neutral lipid stain (582 nm, 616 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | |||||||
| HCS LipidTOX Red phospholipidosis (584 nm, 608 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| HiLyte Fluor™ 405 (403 nm, 428 nm) | ✓✓ | ||||||||||||
| HiLyte Fluor™ 488 (497 nm, 526 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| HiLyte Fluor™ 594 (592 nm, 616 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Hoechst 33258 (532 nm, 455 nm) | ✓✓ | ✓ | |||||||||||
| Hoechst 33342 (352 nm, 455 nm) | ✓✓ | ||||||||||||
| Hoechst 34580 (392 nm, 440 nm) | ✓✓ | ||||||||||||
| Hypericin (600 nm, 603 nm) | ✓ | ||||||||||||
| KFP-Red (575 nm, 599 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | |||||||
| LIVE-DEAD Fixable Green Dead Cell Stain (498 nm, 525 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| LIVE-DEAD Fixable Red Dead Cell Stain (595 nm, 613 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Live-Dead Fixable Violet Dead Cell Stain (403 nm, 455 nm) | ✓✓ | ||||||||||||
| LOLO-1 (568 nm, 580 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Lucifer yellow (428 nm, 544 nm) | ✓ | ✓ | |||||||||||
| LysoSensor Blue (374 nm, 424 nm) | ✓✓ | ||||||||||||
| LysoSensor Green (448 nm, 502 nm) | ✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| LysoTracker Green (501 nm, 509 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| LysoTracker Red (573 nm, 592 nm) | ✓ | ✓ | ✓✓ | ✓✓ | |||||||||
| LysoTracker Yellow HCK-123 (488 nm, 565 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Magnesium Green (507 nm, 531 nm) | ✓ | ✓✓ | ✓ | ||||||||||
| Magnesium Orange (587 nm, 610 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| mApple (566 nm, 594 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ||||||||
| Marina Blue (363 nm, 461 nm) | ✓ | ||||||||||||
| mBBr+GSH (394 nm, 490 nm) | ✓✓ | ✓ | ✓ | ||||||||||
| mCherry (587 nm, 610 nm) | ✓ | ✓✓✓ | ✓✓✓ | ✓ | ✓✓✓ | ||||||||
| Mecrocyanine 540 (559 nm, 579 nm) | ✓ | ✓ | ✓ | ||||||||||
| mHoneyDew (478 nm, 562 nm) | ✓ | ✓ | ✓ | ✓✓ | ✓ | ||||||||
| MitoTracker™ Green (490 nm, 512 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| MitoTracker™ Orange (551 nm, 575 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| MitoTracker™ Red (578 nm, 598 nm) | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | ||||||||
| mKate2 (588 nm, 633 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| mPlum (589 nm,649 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| mRFP (555 nm, 583 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| mRFP1 (584 nm, 700 nm) | ✓ | ✓✓✓ | ✓✓ | ✓ | |||||||||
| mRuby (558 nm, 605 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓ | |||||||
| mStrawberry (574 nm, 596 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓✓ | ||||||||
| mTangerine (568 nm, 585 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| mTFP1 (462 nm, 492 nm) | ✓ | ✓✓ | ✓ | ✓ | |||||||||
| mWasabi (493 nm, 509 nm) | ✓ | ✓✓ | ✓ | ||||||||||
| NBD-X (MeOH) (467 nm, 538 nm) | ✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| NeuroTrace 500/525 Green Fluorescence Nissl Stain (495 nm, 524 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| Nile red-phospholipid (553 nm, 637 nm) | ✓✓ | ✓✓ | ✓ | ✓✓ | ✓ | ||||||||
| Nile red-triglyceride (510 nm, 583 nm) | ✓ | ✓ | |||||||||||
| OFP (547 nm, 567 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Orange 552 (552 nm, 574 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Oregon Green™ 488 (488 nm, 526 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| Oregon Green™ 514 (513 nm, 532 nm) | ✓ | ||||||||||||
| Pacific Blue™ (404 nm, 455 nm) | ✓✓ | ||||||||||||
| PerCP (490 nm, 677 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| pHrodo™, succinimidyl ester (560 nm, 587 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| PicoGreen (502 nm, 522 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| PKH67 (488 nm, 500 nm) | ✓ | ✓✓ | ✓ | ✓ | |||||||||
| POPO-1 (433 nm, 457 nm) | ✓✓ | ✓ | |||||||||||
| PO-PRO-1 (433 nm, 456 nm) | ✓✓ | ||||||||||||
| Propidium Iodide (PI) (305 nm, 617 nm) | ✓✓ | ✓✓ | ✓ | ✓ | ✓ | ||||||||
| Pro-Q Diamond (556 nm, 583 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Qdot® 525 Nanocrystals (300 nm, 525 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Qdot® 545 Nanocrystals (300 nm, 543 nm) | ✓ | ||||||||||||
| Qdot® 585 Nanocrystals (300 nm, 588 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| Qdot® 605 Nanocrystals (300 nm, 602 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Qdot® 625 Nanocrystals (300 nm, 621 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Qdot® 655 Nanocrystals (300 nm, 654 nm) | ✓ | ✓ | ✓ | ||||||||||
| ratiometric pHluorin pH5 (500 nm, 509 nm) | ✓ | ✓ | |||||||||||
| ReAsH-CCPGCC (592 nm, 606 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Red 580 (580 nm, 603 nm) | ✓ | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | |||||||
| Resorufin (571 nm, 584 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Rhod-2 (553 nm, 577 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| Rhodamine 110 (497 nm, 519 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| Rhodamine 123 (507 nm, 529 nm) | ✓ | ✓✓✓ | |||||||||||
| Rhodamine Green (497 nm, 523 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| Rhodamine phalloidin (558 nm, 575 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Rhodamine Red-X (573 nm, 951 nm) | ✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| Rhodol Green (497 nm, 524 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| rsGFP (red shifted GFP, S65T) (498 nm, 516 nm) | ✓✓ | ||||||||||||
| sgBFP™ (387 nm, 451 nm) | ✓✓ | ||||||||||||
| sgGFP™ (super glow GFP) (472 nm, 506 nm) | ✓✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| Sirius (355 nm, 424 nm) | ✓ | ||||||||||||
| SNARF (carboxy) 514 Excitation pH 9 (576 nm, 638 nm) | ✓ | ✓✓ | ✓✓ | ||||||||||
| SNARF-1 488 nm (pH 6.0 ) (548 nm, 586 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| SNARF-1 488 nm (pH 9.0) (576 nm, 638 nm) | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | ||||||||
| SNARF-1 514 nm (pH 6.0 ) (549 nm, 587 nm) | ✓ | ✓✓ | ✓ | ✓ | ✓✓ | ||||||||
| SNARF-1 514 nm (pH 9.0) (576 nm, 638 nm) | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | ||||||||
| Sodium Green (507 nm, 532 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| SpectrumAqua (434 nm, 481 nm) | ✓✓ | ✓ | |||||||||||
| SpectrumBlue (405 nm, 449 nm) | ✓✓ | ||||||||||||
| SpectrumGreen (497 nm, 538 nm) | ✓ | ✓✓ | |||||||||||
| SpectrumOrange (554 nm, 587 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| SpectrumRed (587 nm, 615 nm) | ✓ | ✓ | ✓ | ||||||||||
| Sulforhodamine 101-EtOH (578 nm, 593 nm) | ✓ | ✓✓ | ✓ | ✓✓ | ✓ | ||||||||
| SYBR Gold nucleic acid gel stain-DNA (469 nm, 539 nm) | ✓ | ✓✓ | ✓✓ | ||||||||||
| SYBR Green I nucleic acid gel stain-DNA (498 nm, 522 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| SYBR Safe DNA gel stain-DNA (509 nm, 526 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| SYTO 9 (483 nm, 500 nm) | ✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| SYTO 11 (506 nm, 525 nm) | ✓ | ✓ | ✓✓ | ||||||||||
| SYTO 13 (448 nm, 506 nm) | ✓✓ | ✓✓ | ✓ | ✓ | |||||||||
| SYTO 16 (489 nm, 520 nm) | ✓✓ | ✓✓ | ✓ | ✓✓ | |||||||||
| SYTO 45 (451 nm, 485 nm) | ✓ | ✓✓ | ✓ | ✓ | |||||||||
| SYTO RNASelect green fluorescent cell stain (503 nm, 527 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| SYTOX Blue (444 nm, 470 nm) | ✓✓✓ | ✓ | |||||||||||
| SYTOX Green-DNA (504 nm, 524 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| TagBFP (402 nm, 457 nm) | ✓✓ | ||||||||||||
| TagCFP (458 nm, 480 nm) | ✓✓ | ✓ | ✓ | ✓ | |||||||||
| tdTomato (556 nm, 582 nm) | ✓✓✓ | ||||||||||||
| Tetramethylrhodamine dextran (554 nm, 582 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Texas Red dextran (592 nm, 613 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Texas Red DHPE (584 nm, 608 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Texas Red® (592 nm, 614 nm) | ✓✓✓ | ✓✓ | ✓ | ✓ | |||||||||
| ThiolTracker Violet GSH (404 nm, 526 nm) | ✓ | ✓ | |||||||||||
| TO-PRO-1 (515 nm, 531 nm) | ✓ | ✓ | ✓ | ||||||||||
| TOTO-1 (514 nm, 531 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| TRITC (Tertamethylrhodamine) (552 nm, 578 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| TRITC (Tertamethylrhodamine) - "reddish" (552 nm, 578 nm) | ✓✓ | ||||||||||||
| TurboFP635 (Katushka) (591 nm, 638 nm) | ✓ | ✓ | ✓ | ✓ | |||||||||
| TurboGFP (482 nm, 503 nm) | ✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| TurboRFP (553 nm, 573 nm) | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| TurboYFP (525 nm, 538 nm) | ✓ | ✓ | |||||||||||
| Venus (516 nm, 528 nm) | ✓ | ✓ | ✓ | ||||||||||
| Vybrant DyeCycle Green (506 nm, 534 nm) | ✓ | ✓ | ✓ | ✓✓ | |||||||||
| Vybrant DyeCycle Orange (519 nm, 563 nm) | ✓ | ✓ | |||||||||||
| Vybrant DyeCycle Violet (369 nm, 437 nm) | ✓ | ||||||||||||
| wtGFP (wild type GFP, non-UV excitation) (474 nm, 509 nm) | ✓✓✓ | ✓✓ | ✓✓ | ✓ | |||||||||
| X-Rhod-1 Indicator (580 nm, 601 nm) | ✓ | ✓ | ✓✓ | ✓✓ | ✓✓ | ✓✓ | |||||||
| YFP (yellow GFP) (513 nm, 530 nm) | ✓ | ✓✓✓ | ✓✓ | ||||||||||
| YO-PRO-1 (491 nm, 506 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| YOYO-1 (491 nm, 508 nm) | ✓ | ✓✓ | ✓✓ | ✓ | ✓ | ||||||||
| YOYO-3 (611 nm, 631 nm) | ✓ | ✓ | ✓ | ✓ |
| Posted Comments: | |
Yuan Gong
(posted 2023-12-28 12:08:46.28) Hi,
May I ask about the absorption of the filters, like MF525-39? I would like to buy a pair of this product, and I care about its reflection in the stop band. Can I roughly calculate the reflection at a specific wavelength by R=1-T?
Many thanks! Have a nice one!
Yuan jpolaris
(posted 2024-01-16 04:30:35.0) Thank you for contacting Thorlabs. In general, R = 1-T is not a reliable estimate for reflectance in the stop band because it ignores the effects of absorption and interference which could be part of the blocking mechanism. I have reached out to directly with reflectance data. Priyanka Jangra
(posted 2023-09-13 22:19:46.323) We want to know about the product FMF003 availability and price jdelia
(posted 2023-09-13 01:33:48.0) Thank you for reaching out. FMF003 was obsoleted and replaced by MF535-22. You can find pricing and availability information for this product directly on the product family page. Kevin Cappa
(posted 2020-03-04 17:50:33.503) If I had the option to get a 2"/50mm filter set for FITC fluorescent imaging I would... nbayconich
(posted 2020-03-05 01:03:35.0) Thank you for your feedback, I will log your request in our internal engineering forum for future reference. We can provide custom sized filters upon request, I will reach out to you directly to discuss our custom capabilities. tcohen
(posted 2012-09-13 11:44:00.0) Response from Tim at Thorlabs: Thank you for your interest in our custom capabilities. We should be able to provide this as a special and I have contacted you to go over your requirements. densta
(posted 2012-09-13 12:33:11.0) Dear Madam/Sir
I want to perform some preliminary fluorescence tests for a project of ours.
I am interested in buying one of the following emission filters
MF525-39: GFP
MF530-43: FITC
I noticed from your records that the outer diameter of the filter is 25 mm.
In our system we need a filter with outer diameter 17 mm. The filter could be bare glass without an outer support ring. Is it possible to have one of the above mentioned filters in this form? What would be the cost?
Best regards,
Dimosthenis Stamopoulos bdada
(posted 2012-02-24 17:13:00.0) Response from Buki at Thorlabs to
Thank you for your interest in our imaging dichroic filters.
Testing has shown no signs of degradation when exposed to at least 6 W of power from an unfiltered xenon arc lamp over a 25 mm diameter (corresponding to 1.2 W/cm2) for over 500 hours.
Please contact TechSupport@thorlabs.com if you have any questions. user
(posted 2012-02-10 13:05:04.0) Can you please provide laser damage threshold ratings for the dichroic filters? jjurado
(posted 2011-03-22 16:05:00.0) Response from Javier at Thorlabs to flickingerd: Thank you for your feedback. Our filters follow that same convention as that of Semrock's. The arrow points to the recommended direction of light propagation. The reason for this is although the filter will function with either side facing the source, it is recommended to place the coated side toward the source. This will minimize any thermal effects or possible thermal damage that blocking intense out-of-band radiation might cause due to absorption by the substrate or colored glass filter layers. lmorgus
(posted 2011-03-21 18:23:00.0) A response from Laurie at Thorlabs to flickingerd: As a follow-up to the response you received earlier today from Javier in our technical support group, I wanted to also reach out to you to thank you for your feedback. We have updated our web presentation to provide information about what the arrow on these optic mounts indicates. As a further step, we have made a list of other web pages on our site that need some attention for similar reasons. In the coming days, we will be updating those pages to clarify our engravings so that our customers can efficiently find the information they need to use our products effectively. Again, thank you for taking the time to drop us a note with a suggestion for improvement. flickingerd
(posted 2011-03-21 14:29:07.0) I couldnt find info on proper filter orientation. There are arrows on the filters, but Ive seen different conventions on what these mean (Chroma says point arrow towards specimen for emission filters, yet Semrock convention is always point arrow in direction of light propagation, I think). How about your filters? This should be in "Tutorial" section, Id think. Please contact me with proper orientation rules. (Although Im still not sure exactly why this matters...I guess I can think of some minor reasons...) apalmentieri
(posted 2010-02-18 09:42:22.0) A response from Adam at Thorlabs to pludowise: I believe we should be able to offer this item as a custom option. I would like to contact you directly to get more information about your application, the quantity necessary, and the exact size of the optic. pludowise
(posted 2010-02-18 00:01:38.0) I am interested in finding a dichroic filter similar to MDF-TXRED, but shifted to loger wavelengths. Specifically, I need a dichroic for a Cy5 dye, which needs to transmit at 640 nm, and reflect at 690 nm. I also need this in a 1 mm thickness mounted at 45 deg AOI. Thank you. |

These excitation fluorescence imaging filters are specifically designed to be used in microscopy and imaging applications. Each Ø25 mm filter is mounted in a 5 mm thick black anodized housing. The housing has an arrow engraved on it that points in the recommended light propagation direction.
These filters provide excellent transmission of the desired excitation wavelength (>90%), with a sharp spectral cutoff and low transmission at other wavelengths (<0.001%). Click on the ![]() icons above to view product-specific transmission data. For additional fluorophore compatability, please see the Fluorophores tab.
icons above to view product-specific transmission data. For additional fluorophore compatability, please see the Fluorophores tab.

These emission fluorescence imaging filters are specifically designed to be used in microscopy and imaging applications. Each Ø25 mm filter is mounted in a 3.5 mm thick black anodized housing. The housing has an arrow engraved on it that points in the recommended light propagation direction.
These filters provide excellent transmission of the desired emission wavelength (>90%), with a sharp spectral cutoff and low transmission at other wavelengths (<0.001%). Click on the ![]() icons above to view product-specific transmission data. For additional fluorophore compatability, please see the Fluorophores tab.
icons above to view product-specific transmission data. For additional fluorophore compatability, please see the Fluorophores tab.

Thorlabs' Dichroic Filters are designed to separate light of different wavelengths. When light is incident on the filter at a 45° angle with respect to the normal, the excitation light and its associated back reflection are reflected while the longer wavelength fluorescence signal is transmitted. These filters are unmounted, but are marked by a dash to indicate the coated side of the filter, which light should be incident on. Each filter is 25.0 mm x 36.0 mm. If your application would benefit from a round, mounted dichroic filter, consider our round Dichroic Filters. Click on the ![]() icons above to view product-specific transmission data. For further fluorophore compatability, please see the Fluorophores tab.
icons above to view product-specific transmission data. For further fluorophore compatability, please see the Fluorophores tab.

Since standard fluorescence imaging applications generally incorporate three different filters (i.e., one excitation, one emission, and one dichroic filter) to maximize the signal-to-noise ratio, Thorlabs offers these filters as a set at a savings over purchasing them separately.
Please Note: The excitation and emission filter housings have an arrow engraved on them that points in the recommended direction of light propagation. Dichroic filters have a marking on the side with the beamsplitter coating. Light should be incident on the marked side of the optic for optimal performance. For further fluorophore compatability, please see the Fluorophores tab.
 Products Home
Products Home



















 Click to Enlarge
Click to Enlarge
 Zoom
Zoom


 Fluorescence Imaging Filters
Fluorescence Imaging Filters