Second Harmonic Generation in Multiphoton Microscopy
Featured Researchers:
Thorlabs Virginia R&D Team
|
Nonlinear optical microscopy (NLOM) is a well established tool for the interrogation of living biological specimen. The multitude of imaging techniques (two- and three-photon excited fluorescence, second and third harmonic generation) that encompass nonlinear microscopy allows the collection of structural, functional and chemical information at imaging depths of hundreds of microns within the sample. Advances in hardware, optics, and laser sources expand upon the wealth of data already available to scientists by facilitating experiments with faster imaging speeds over longer time periods at deeper imaging depths.
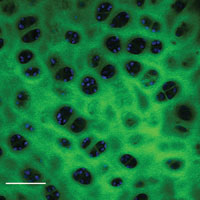
Figure 2.
Cross section of chicken tibia. Green pseudo color indicates second harmonic generation from collagenous fibers within the bone. Blue pseudo color is autofluorescence of osteoblasts within the trabeculae. Scale bar 100 µm.
Imaging depth in NLOM is primarily limited by the scattering of laser light into the sample and signal coming out of the sample. In biological specimen this attenuation is heavily dominated by Mie-Scattering, which is inversely proportional to λ4. By shifting the excitation wavelength and signal (fluorescence or harmonic generation) wavelengths further to the red, imaging depth can be increased without the need for regenerative amplifier laser sources, which are not compatible with fast imaging speeds.
A particular advantage to NLOM in live tissue imaging is the sensitivity to endogenous contrast mechanisms without relying on ultraviolet excitation. Second harmonic generation requires specific organizational parameters of biological components in order to be observed. The most common constituent of biological tissue that satisfies these conditions is collagen. Figure 1 shows second harmonic generation from collagen in chicken leg tendon. Although each image was taken at the same focal plane, the incident polarization was rotated as indicated by the double ended arrow, using an achromatic half-wave plate. As the incident laser polarization is rotated (refer to Figs. 1A and 1B), greater signal strength can be seen from collagen fibrils that are better oriented with the incident polarization. The signal in Figure 1C is obviously reduced compared to A&B as evidenced by the poor alignment of the incident polarization to any collagen fibers. The signal dependence as a function of fibrillar orientation can then provide an additional level of information on cellular, mechanical, or environmental interactions that dictate collagen organization.
Second harmonic generation can also be observed in skeletal muscle and microtubules. Through two- and three-photon excited fluorescence the identification of particular cellular structures with distinct spectral emission characteristics depending on function can be observed. For example, two photon excitation fluorescence images of NAD(P)H, retinol, or flavins can provide functional chemo-biological information or be used as structural markers. Figure 2 shows a combined image of second harmonic generation from chicken bone and two-photon excited autofluorescence of osteoblasts within the trabeculae of chicken tibia. Two-photon excitation fluorescence can also provide signal from extracellular structures such as elastin.
 (c)  (b)  (a) Figure 1.
Second harmonic generation from chicken leg tendon at the interface of the connective tissue around the collagen fiber bundle. The connective tissue has a radial orientation of the collagen fibers as compared to the collagen fibril with a longitudinal orientation. The red arrows indicate the change in signal for vertically oriented fibers. The yellow arrows indicate the change in signal for longitudinally oriented fibers. Scale bar 100 µm.

|
The images shown here were taken with Thorlabs' Video-Rate Multiphoton Microscope and a 20X 1.0 NA water immersion objective. A femtosecond Ti:Sapphire laser tuned to 860 nm was used as the excitation source. Images are 1024 x 1024 pixels taken at a rate of 14 fps.




 Products Home
Products Home
 Multiphoton Second Harmonic Generation
Multiphoton Second Harmonic Generation
Response from Sean at Thorlabs: The six images at the top of the page are an overview of images taken using multiphoton microscopy.
Top Rrow:
Left: Two-photon fluorescence of DAPI (blue), AlexaFluor488 (green) and AlexaFluor568 (red) in the mouse kidney. Image obtained using the Thorlabs Multiphoton Microscope with an Olympus 20X 1.0 NA objective.
Middle: Multiphoton imaging of GFP fluorescence in dopamine receptors in C. Elegens. Olympus 20X 1.0 NA W. Sample provided by William Ryu, University of Toronto.
Right: Two-color mouse liver image.
Bottom Row:
Left: Mouse embryo section, sample courtesy of Dr. Rieko Ajima, National Cancer Institute, Center for Cancer Research.Middle: One-color fluorescence microscopy image of a mouse kidney.
Right: Mouse Embryo Section, Sample Courtesy of Dr. Rieko Ajima, National Cancer Institute, Center for Cancer Research.
Many of these images can be seen here in larger sizes: http://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=5309